Medical-Grade Polycaprolactone (PCL) is a semi-crystalline, aliphatic polyester critical to the advancement of biodegradable polymers in the healthcare sector. Characterized by its low melting point and extended degradation profile, PCL offers a material platform demanding sustained structural integrity prior to bioresorption. Its inherent biocompatibility and adjustable mechanical properties support applications spanning porous tissue engineering scaffolds, complex implantable devices, and controlled-release drug delivery systems.
Successfully implementing PCL requires a profound understanding of the material’s precise attributes and the increasingly stringent regulatory compliance required for “Medical-Grade” designation. This guide provides a foundational and detailed examination of the fundamental properties, specific processing considerations, and essential compliance standards that position Medical-Grade PCL as a leading material solution in modern medical technology.
Molecular Structure and Fundamental Properties
Medical-Grade Polycaprolactone (PCL) is a semi-crystalline, linear aliphatic polyester synthesized through the ring-opening polymerization of ε-caprolactone. Its molecular backbone consists of repeating hexanoate units, defined by the chemical formula (C6H10O2)n. This specific structure provides the unique combination of physical and mechanical characteristics essential for clinical utility.
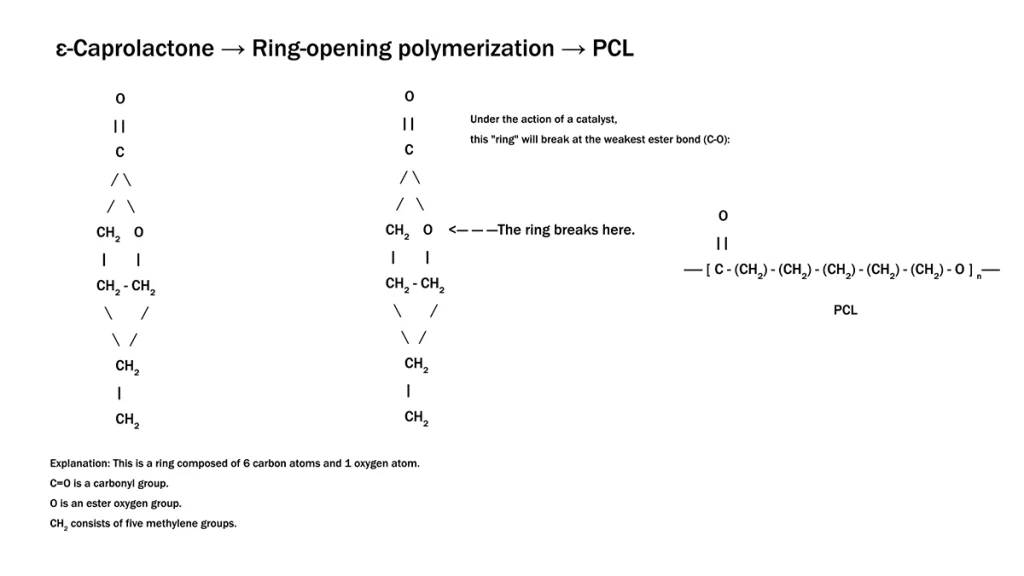
Molecular structure of Polycaprolactone showing the repeating hexanoate units formed through ring-opening polymerization
Thermal Properties
PCL is characterized by its exceptionally low glass transition temperature (Tg), typically ranging from -65°C to -60°C, which ensures the material remains flexible and elastic at physiological temperatures.Simultaneously, its low melting point (Tm) of 59℃ to 64℃ is a key advantage, facilitating excellent processability through methods like fused deposition modeling (3D printing) and injection molding with minimal risk of thermal degradation. The material exhibits a semi-crystalline nature, often with a crystallinity between 40% and 60%, and a typical density of 1.14g/cm³. Medical-grade PCL is manufactured with controlled molecular weights, commonly ranging from 10,000 to 80,000 g/mol, directly influencing its mechanical strength and bioresorption rate.
Mechanical Characteristics
PCL offers considerable mechanical versatility. It presents a typical tensile strength of 10-35 MPa and high elongation at break, reaching 300-600%. This balance of strength and inherent elasticity is crucial for dynamic tissue interfaces. The material’s relatively low Young’s modulus (approximately 400 MPa) enhances its compliance with soft biological tissues, minimizing stress shielding. Furthermore, the viscoelastic properties of PCL can be precisely tailored through molecular weight adjustments and specific processing techniques to meet demanding device specifications.
| Property | Value Range | Significance in Medical Applications |
| Molecular Weight (Mn) | 10,000-80,000 g/mol | Direct control over degradation rate and mechanical performance. |
| Glass Transition Temperature (Tg) | -65°C to -60°C | Guarantees polymer flexibility at body temperature. |
| Melting Point (Tm) | 59°C to 64°C | Enables low-temperature, energy-efficient processing. |
| Young’s Modulus (E) | ~ 400 MPa | Provides compliance with soft tissue interfaces. |
Biodegradability and Degradation Pathway
The controlled biodegradability of Medical-Grade Polycaprolactone (PCL) is its defining feature for long-term medical applications. PCL’s slow and predictable breakdown in physiological environments ensures the material’s structural role is maintained until the target tissue has healed or regenerated, gradually converting into biocompatible products that the body can metabolize or excrete.
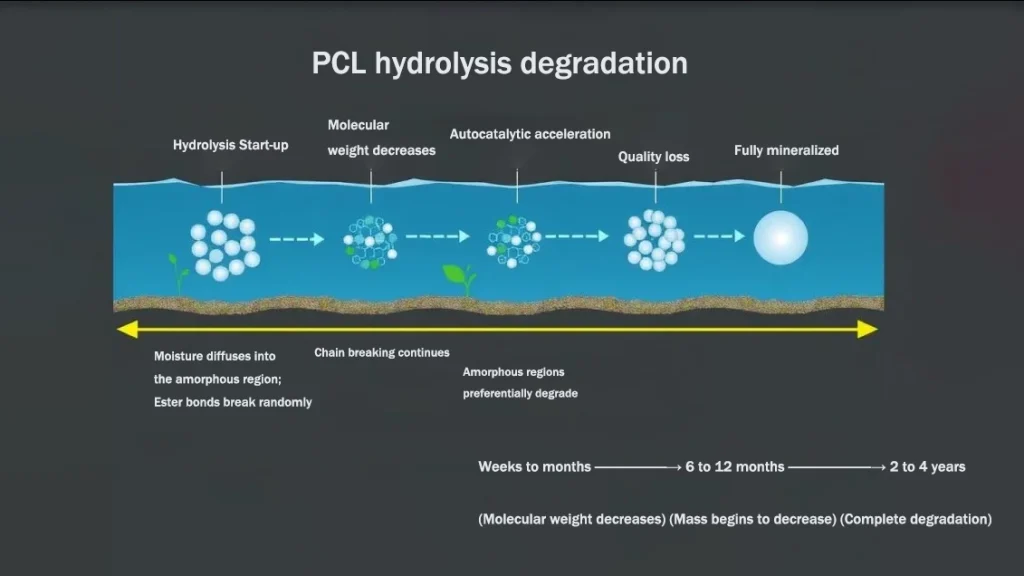
Visualization of PCL’s hydrolytic degradation pathway showing progressive breakdown of polymer chains over time
Mechanism and Rate Factors
PCL degradation proceeds primarily via hydrolytic cleavage of the ester bonds in its polymer backbone. This bulk erosion initiates in the amorphous regions and progresses toward the more resistant crystalline domains. The rate of hydrolysis is highly tunable by material and environmental parameters:
- Molecular Weight: Higher molecular weight significantly slows the degradation rate.
- Crystallinity: Higher crystallinity increases resistance to water penetration, leading to a slower breakdown.
- Surface Area to Volume Ratio: Increased surface area accelerates the hydrolytic exposure and subsequent degradation.
- Environmental Factors: Local pH and temperature variations can influence the reaction kinetics.
Timeline and Metabolic Fate
PCL exhibits an extended degradation profile, typically requiring 2 to 4 years for complete resorption in vivo. This slow timeline is optimal for long-term implantable devices and scaffolds where sustained mechanical support and gradual tissue integration are necessary. The ultimate degradation product, 6-hydroxycaproic acid, is a non-toxic intermediate that is readily channeled into the Tricarboxylic Acid (TCA) cycle, where it is fully metabolized into carbon dioxide and water. This metabolic fate contributes significantly to PCL’s excellent biocompatibility, preventing the localized buildup of acidic byproducts that commonly provoke inflammatory responses with other fast-degrading polymers.

Medical-Grade PCL: Specialized Attributes and Compliance
The designation of Medical-Grade Polycaprolactone is fundamentally defined by strict quality control and comprehensive regulatory compliance that significantly differentiates it from industrial counterparts. This standard extends beyond inherent material properties to encompass purity, manufacturing consistency, and full traceability required for clinical implementation.

Clean room manufacturing facility for medical-grade PCL production under controlled conditions
Purity and Biocompatibility
Medical-grade PCL must adhere to exceptionally high purity specifications, typically exceeding 99.5%. Critical contaminants are controlled to precise limits to ensure product safety. These limits include restricted levels of residual monomers (e.g., ≤ 0.1% via GC-MS), catalysts, and processing aids. Furthermore, heavy metal content is stringently limited to parts-per-billion, and endotoxin levels must meet standards (e.g., ≤ 0.5 EU/g via LAL Test) to prevent pyrogenic reactions in vivo. These materials undergo mandatory biological evaluation and testing in accordance with the ISO 10993 series for cytotoxicity, sensitization, and systemic toxicity.
Manufacturing Control and Traceability
Production must occur under controlled environments compliant with ISO 13485 and cGMP (current Good Manufacturing Practice) quality management systems. This ensures consistent batch-to-batch properties, including controlled molecular weight distribution (PDI ≤ 1.8). Every batch is accompanied by a detailed Certificate of Analysis (CoA) documenting key parameters (Mw, Tm, crystallinity). Crucially, full lot traceability is maintained from raw material sourcing through final packaging, providing the necessary audit trail for quality assurance and regulatory submission protocols.
Sterilization Compatibility
Maintaining critical material integrity post-sterilization is mandatory for implantable devices. PCL exhibits compatibility with common methods, though considerations are required:
- Ethylene Oxide (EtO): Generally preferred, demonstrating minimal impact on PCL’s core chemical and mechanical properties.
- Gamma and E-beam Irradiation: These methods may induce polymer chain scission, which can consequently alter the material’s molecular weight and accelerate its expected degradation rate.
- Steam Autoclaving: Typically avoided due to PCL’s low melting point (59℃-64℃), which risks deformation.
Regulatory Support
Manufacturers of Medical-Grade PCL provide comprehensive technical and regulatory documentation critical for facilitating the finished medical device approval process. This support often includes the submission of a Drug Master File (DMF) or Device Master File to FDA, along with Technical Files and declarations of conformity necessary for compliance with EU MDR (Medical Device Regulation) and other international standards.
Medical-Grade vs Industrial-Grade PCL: Comparative Analysis
The distinction between medical-grade and industrial-grade PCL is defined by the rigorous protocols employed to mitigate risk in clinical settings. This contrast lies in the manufacturing process controls, validated purity standards, and robust regulatory documentation essential for products contacting human tissues. For any application, appropriate material specification is paramount.

Visual comparison of medical-grade PCL (left) with pharmaceutical packaging and industrial-grade PCL (right) highlighting differences in presentation and documentation
Medical-Grade PCL
Medical-Grade PCL is processed to meet the stringent demands of patient safety and regulatory bodies:
- Manufactured under ISO 13485 Quality Management System and cGMP compliance.
- Features stringent impurity control, with residual monomer levels strictly limited (≤ 0.1%).
- Is Endotoxin certified (≤ 0.5 EU/g) to prevent pyrogenic reactions.
- Guarantees consistent lot-to-lot properties, defined by a narrow Mw specification and low polydispersity index (PDI ≤ 1.8).
- Supported by a full ISO 10993 biocompatibility file, Stability Studies, and complete lot traceability.
- Regulatory support is available via existing DMF or Technical Files.
- Mandatory for all long-term or direct body contact applications (e.g., implants, tissue scaffolds).
Industrial-Grade PCL
Industrial-Grade PCL is suitable only for non-medical uses where regulatory scrutiny and direct patient contact are not factors:
- Production occurs under standard industrial quality conditions; no ISO 13485 or cGMP compliance is required.
- Exhibits higher impurity tolerance, with endotoxin testing typically absent.
- Features a wider specification range and variable Mw and PDI between batches.
- Only provides a basic Certificate of Analysis (CoA) and minimal lot traceability.
- Regulatory support documentation is minimal or unavailable.
- Appropriate only for non-critical uses (e.g., prototyping, adhesives, packaging).
Key Applications in Medical and Surgical Fields
Medical-Grade Polycaprolactone (PCL) has established itself as a cornerstone biomaterial due to its unique combination of biocompatibility, controlled degradation, and favorable mechanical properties. Its versatility allows for deep customization, making it particularly valuable in fields requiring long-term implantable materials that facilitate gradual tissue integration.
Tissue Engineering and Regenerative Medicine
PCL’s extended degradation timeline and excellent processability into complex porous structures support gradual tissue regeneration across orthopedic and soft tissue applications:
Bone Tissue Engineering
PCL scaffolds provide essential structural support aligned with the slow timeline of bone healing and remodeling. Composites incorporating bioactive ceramics (e.g., hydroxyapatite or β-tricalcium phosphate) further promote osteoblast adhesion and differentiation, enhancing bone integration.

Cartilage Regeneration
PCL’s mechanical characteristics can be tailored to match the viscoelastic nature of native cartilage. It serves as an optimal substrate for chondrocyte growth and matrix deposition, with applications ranging from articular cartilage to meniscus repair.
Advanced Drug Delivery Systems
PCL’s ability to achieve controlled diffusion and its tunable degradation profile make it an ideal polymer for advanced pharmaceutical delivery:
Microspheres and Nanoparticles
PCL is widely formulated into micro- and nanoparticles for injectable depot systems, enabling sustained release of therapeutics (including antibiotics, growth factors, and chemotherapeutics) over weeks or months, reducing necessary dosing frequency.

Implantable Reservoirs
PCL can be fabricated into non-eroding or slowly degrading implantable devices that provide localized, long-term release of therapeutic agents directly to the target site. Key examples include intravitreal implants for retinal diseases and localized systems for chronic pain or infection control.

Surgical and Implantable Devices
The mechanical strength and eventual bioresorption of PCL are leveraged in several surgical contexts requiring temporary support:
Surgical Sutures
PCL monofilaments and multifilaments function as absorbable suture materials characterized by extended strength retention (typically 3–6 months). This makes them critically suitable for slow-healing tissues like tendons and fascia, where premature loss of tensile strength is unacceptable.
Surgical Meshes and Barriers
PCL meshes provide temporary mechanical reinforcement for soft tissue repair (e.g., hernia repair). As barriers for guided tissue regeneration, they offer structural integrity while gradually transferring load to the regenerating tissue as the material is slowly resorbed.
Explore Application-Specific PCL Formulations
Discover our specialized Medical-Grade PCL formulations optimized for tissue engineering, drug delivery, or surgical applications.
Advantages and Considerations for Medical Use
A balanced assessment of Medical-Grade Polycaprolactone (PCL) is crucial for successful material selection. Evaluating its comparative advantages against inherent challenges allows developers to leverage PCL’s unique strengths while implementing targeted strategies to achieve optimal clinical performance.
Advantages
PCL possesses several distinguishing benefits that position it favorably against other biodegradable polymers:
- Controlled Degradation: Extended timeline of 2–4 years, ideal for long-term implant integration.
- Biocompatibility and Safety: Degradation results in neutral products, avoiding acidic microenvironments common with PLA/PGA.
- Superior Processability: Low melting point (~ 60℃) supports energy-efficient processing and advanced techniques like 3D printing.
- Versatility: Excellent compatibility for blending and material property customization.
- Regulatory History: Established FDA approval history, streamlining regulatory pathways.
Considerations
Potential challenges must be addressed during device development:
- Mechanical Strength: Insufficient inherent strength for certain high-load bearing applications without reinforcement.
- Surface Hydrophobicity: Hydrophobic surface often hinders initial cell attachment.
- Sterilization Limits: Low melting point requires caution; radiation may induce chain scission, and steam autoclaving is avoided.
- Cost: Represents a higher initial material cost compared to standard polymers.
Strategies for Optimization
These limitations can be effectively addressed through established material science techniques:
- Surface Modification: Use plasma treatment or protein coating to enhance hydrophilicity and cell interaction.
- Composite Formulations:
- PCL/Ceramic: For enhanced osteoconductivity and mechanical modulus (e.g., HA).
- PCL/Copolymer: For precisely tailoring the final degradation rate (e.g., PCL/PLA).
Cost-Benefit Analysis
The total value of Medical-Grade PCL offsets the initial material cost through critical long-term advantages:
- Compliance and Risk: Comprehensive documentation accelerates approval timelines and reduces compliance costs.
- Clinical Performance: Predictable, safe degradation minimizes adverse clinical events and associated liability.
- Manufacturing Efficiency: Lower processing temperatures translate into potential energy and capital savings.
Future Trends and Innovations
The trajectory of Medical-Grade Polycaprolactone (PCL) development is accelerating, driven by its transition from a passive biodegradable material to an active, functional component in advanced therapeutic systems. Several key trends at the intersection of materials science and bioengineering are defining the future of PCL in medicine.
Emerging Research Directions
Research is focused on enhancing PCL’s capability to interact dynamically with the biological environment:
- Smart and Responsive Systems: Development of PCL materials that respond to physiological cues (e.g., pH, temperature, enzymes), enabling self-regulating systems and shape-memory implants.
- Bioactive Derivatives: Chemical modification to incorporate inherent bioactivity, such as antimicrobial peptides or cell-signaling molecules, actively directing cellular responses.
- Nanofunctionalized Composites: Integration of nanoparticles (e.g., silver, iron oxide) to create multifunctional PCL systems with imaging or antimicrobial capabilities.
Technological Advancements
Innovations in manufacturing and design tools are expanding the complexity and precision of PCL devices:
- Advanced Manufacturing Techniques: Utilizing multi-material bioprinting and 4D printing to create intricate, patient-specific scaffolds that change shape or function over time.
- Computational Design and Modeling: Employing Finite Element Analysis (FEA) and Computational Fluid Dynamics (CFD) to predict performance, along with digital twins for personalized prediction.
Regulatory and Market Evolution
The market landscape is maturing, supporting wider adoption and specialization:
- Standardization and Guidance: Continuous harmonization of international standards, including specific regulatory guidance for 3D-printed PCL devices.
- Market Specialization: Growing demand for specialized, application-specific PCL formulations tailored for niche areas like retinal implants or personalized orthopedic regeneration.
The future of medical-grade PCL lies at the intersection of materials science, advanced manufacturing, and computational design. This convergence promises unprecedented capabilities in creating functional, responsive biomaterials that actively participate in the healing process.
Call to Action
Medical-Grade Polycaprolactone (PCL) has proven its capability as an essential platform for advancing medical device technology, tissue engineering solutions, and controlled drug delivery systems. The material’s unique combination of controlled, non-acidic biodegradability, processing versatility, and established regulatory history provides a robust foundation for innovation.
Realizing the full potential of PCL in clinical applications requires securing a supply that meets the highest standards for quality, consistency, and traceability. We stand ready to support researchers and medical product developers by providing fully compliant, medical-grade PCL specifications, ensuring material integrity and regulatory confidence throughout the entire product lifecycle.
Advance Your Medical Innovation with Expert PCL Consultation
Our team of materials scientists and regulatory specialists can provide comprehensive guidance on incorporating PCL into your medical product development process.

Our interdisciplinary team of scientists and engineers specializes in PCL-based medical material development and regulatory guidance
Frequently Asked Questions
Q:What temperature does PCL melt at?
A:Polycaprolactone typically melts between 59℃ and 64℃, with medical-grade specifications often focusing on a narrow range (e.g., 60℃–62℃). This low melting temperature provides excellent processability at moderate temperatures while ensuring thermal stability well above physiological temperature (37℃).
Q:How to dissolve polycaprolactone?
A:PCL is soluble in various organic solvents at room temperature, including chloroform, dichloromethane (DCM), and acetone. For medical applications, the choice of solvent must be followed by rigorous removal procedures to ensure residual solvent levels comply with regulatory requirements, such as the ICH Q3C guidelines (typically ≤ 50 ppm for Class 2 solvents).
Q:What are the thermal properties of polycaprolactone?
A: PCL exhibits a very low glass transition temperature (Tg) of approximately -60℃ to -65℃ and a melting point (Tm) of 59℃ to 64℃. Its semi-crystallinity ranges from 40% to 60%. These properties ensure the material remains flexible (Tg < 37℃) while maintaining structural integrity at body temperature.


