Polylactic Acid (PLA) is firmly established as a foundational biomedical polymer, recognized globally for its derivation from renewable resources and its clinically essential attributes: biocompatibility and tunable degradation kinetics via controlled hydrolysis. This thermoplastic polyester’s performance in high-stakes clinical applications—spanning advanced medical device scaffolds, absorbable sutures, and precision drug delivery systems—is fundamentally governed by its stereochemical composition (e.g., PLLA, PDLA, PDLLA).
Addressing the dual demands of rigorous clinical performance and the growing regulatory focus on sustainable healthcare, a precise understanding of the specific performance metrics and polymer grades is essential for successful medical application development and regulatory compliance.
Core Technical Requirements for Medical-Grade PLA Raw Materials
Medical-grade Polylactic Acid (PLA) raw materials are fundamentally differentiated from commodity and food-grade variants by stringent technical specifications that mandate ultra-high purity and batch-to-batch consistency. These are non-negotiable prerequisites for clinical safety, efficacy, and predictable in vivo performance. The polymer must meet far stricter requirements than those used in packaging, necessitating specialized synthesis and advanced purification to eliminate trace contaminants, precisely control specific molecular characteristics, and guarantee a predictable hydrolytic degradation profile.
Critical Technical Parameters for Implantation
The clinical utility of PLA is a direct function of the raw material quality. The critical technical parameters outlined below summarize the tight tolerances necessary for reliable processing and optimal patient outcomes:
| Parameter | Specification | Clinical Significance |
| Molecular Weight (Mw) | 10,000 – 300,000 Da (Application-Specific) | Directly dictates mechanical strength, viscosity during processing, and in vivo degradation rate. |
| Optical Purity | ≥ 98% for PLLA/PDLA Homopolymers | Controls polymer crystallinity, thermal stability, and ultimate mechanical properties (e.g., tensile modulus). |
| Residual Monomer Content | ≤ 0.5% (Lactide) | Minimizes potential cytotoxicity and ensures a consistent, predictable hydrolytic degradation pathway. |
| Heavy Metal Content | ≤ 10 ppm Total | Mitigates long-term systemic toxicity risk in implantable devices. |
| Endotoxin Level | ≤ 0.5 EU/g | Prevents inflammatory or pyrogenic responses in the patient following implantation. |
| Biocompatibility | USP Class VI, ISO 10993 Compliance | Verifies material safety regarding cytotoxicity, sensitization, irritation, and systemic effects. |
The consistent production of these high-specification materials mandates operation within certified cGMP (current Good Manufacturing Practices) environments. Crucially, a robust quality management system ensures full batch traceability and comprehensive Release Testing, providing the necessary assurance that every kilogram of raw material conforms to the required regulatory and performance standards.
Stereochemistry and Application-Specific PLA Grades
The diverse array of physical, chemical, and degradation properties available through different PLA grades (PLLA, PDLA, PDLLA, and PLGA copolymers) enables its successful utilization across four critical high-end biomedical sectors. The specific choice of stereoisomer critically determines the final product performance:
- Poly-L-lactic Acid (PLLA): Highly crystalline and strong; used for long-term structural applications (e.g., orthopedic fixation).
- Poly-D,L-lactic Acid (PDLLA): Amorphous (non-crystalline) and weaker; used for fast-degrading or amorphous applications (e.g., drug delivery microspheres).
Orthopedic implants manufactured from high-molecular-weight PLLA showing excellent mechanical properties
Orthopedic Implants
This field demands polymers with high mechanical integrity and controlled resorption. High-molecular-weight PLLA formulations are essential for load-bearing devices, providing the required initial strength. The material facilitates the gradual transfer of mechanical stress to the healing bone over 18-36 months, eliminating the need for invasive secondary removal procedures.
- Bone screws and pins requiring specific strength retention curves.
- Fixation plates engineered with tailored modulus and stiffness.
- Interference screws for ligament and tendon reconstruction.
- Suture anchors optimized for stable pull-out strength in bone.
Drug Delivery Systems
PLA and its copolymer PLGA are cornerstones for advanced drug delivery, where precise control over the release profile is paramount. The polymer composition (stereo-purity and lactide-to-glycolide ratio) allows for the fine-tuning of the degradation rate, thus regulating the elution of active therapeutic agents over predefined intervals.
- Microspheres and injectable depots for sustained release of peptides and small molecules.
- Nanoparticles utilized for enhanced target specificity in chemotherapy.
- Implantable matrices designed for localized, long-term drug elution.
- Injectable in-situ forming implants for non-invasive drug administration.
Tissue Engineering Scaffolds
In regenerative medicine, PLA-based scaffolds provide the three-dimensional bio-architectures required for cellular attachment, proliferation, and subsequent tissue regeneration. The material’s versatility permits the engineering of specific structural characteristics, including controlled porosity, surface topography, and mechanical compliance to guide de novo tissue formation.
- 3D-printed, patient-specific scaffolds for defect repair.
- Electrospun nanofiber matrices mimicking the native extracellular matrix.
- Porous structures with designed interconnectivity and controlled degradation.
- Bioactive composite scaffolds integrated with cell signaling factors.
Medical Devices and Components
Medical-grade PLA’s excellent thermal processability—via techniques like high-precision injection molding, extrusion, and additive manufacturing—makes it ideal for a broad spectrum of disposable and temporary devices. The material offers a reliable, biocompatible alternative in products where a defined service life is required.

Medical devices manufactured from PLA including microneedles and precision components
Short-Term Applications
Surgical instruments and accessories, specialized diagnostic components, and dissolvable wound closure devices.
Long-Term Applications
Absorbable stents with programmed radial strength loss and nerve guidance conduits requiring specific degradation windows.
Market Strategy: Why Now is the Best Window of Opportunity to Enter the Medical PLA Market
The current market represents a critical strategic window, characterized by intense adoption drivers and high entry barriers that prioritize raw material expertise. Global demand is accelerating due to stringent regulatory pressures—specifically the mandated substitution of traditional polymers containing problematic additives like BPA and phthalates (EU MDR, etc.)—and widespread healthcare sustainability mandates.
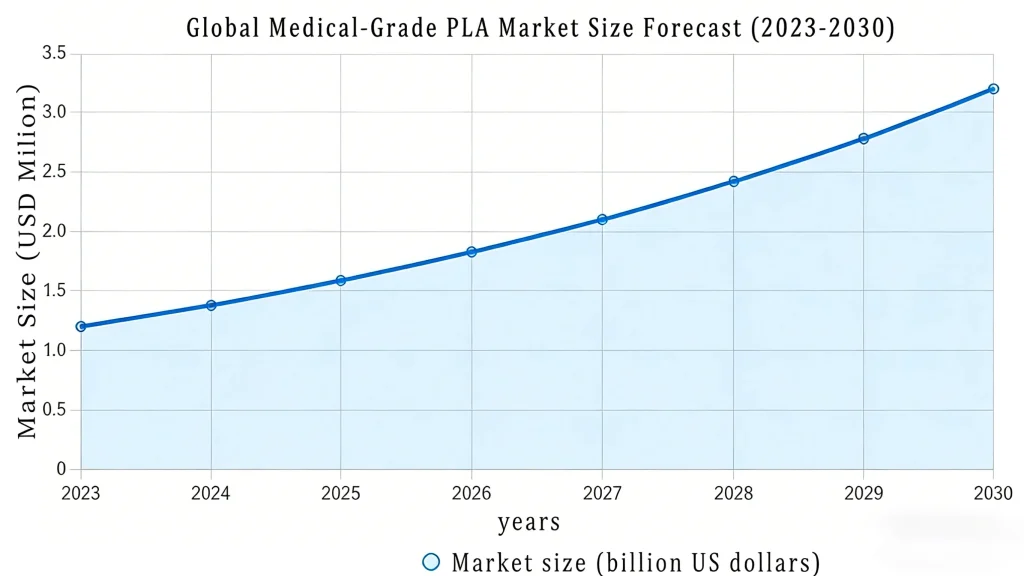
PLA medical market growth projections (2023-2030) showing regulatory milestones and adoption trends
Market Drivers
- Regulatory Pressure: Increasing restrictions on traditional petroleum-based plasticizers and additives.
- Sustainability: Healthcare systems adopting strict carbon reduction and bio-based sourcing goals.
- Technological Maturity: Advancements enabling complex PLLA/PLGA-based devices (e.g., 3D printing, electrospinning).
Strategic Challenges
- Regulatory Complexity: Extended approval timelines for novel, application-specific biomaterials.
- Performance Perception: Need to overcome lingering performance misconceptions regarding mechanical robustness.
- Manufacturing Expertise: Limited access to specialized knowledge for cGMP synthesis and precision processing.
Market Opportunity Assessment
Securing an advantageous position now hinges on mastering the technical barriers—namely, delivering the high-purity, batch-consistent raw material grades required—to capitalize on this imminent market acceleration.
Strategic Sourcing and Supply Chain Reliability
A reliable and compliant raw material supply chain constitutes a non-negotiable risk management step for medical device manufacturers utilizing PLA. Highly specialized medical-grade polymers demand suppliers function as true partners, which requires both robust technical competency and established regulatory infrastructure. Ensuring material integrity from synthesis through final delivery necessitates a rigorous, multi-faceted supplier qualification process integral to strategic sourcing.

Medical-grade PLA manufacturing facility operating under cGMP conditions with comprehensive quality control
Key Supplier Qualification Criteria
Supplier due diligence must focus on documented proof of quality and consistency:
Quality System Compliance
- ISO 13485 certification for quality management systems.
- Adherence to cGMP manufacturing environments.
- Robust change control and notification procedures.
- Provision of Master Files and comprehensive biocompatibility data (ISO 10993).
Technical Delivery Capability
- Consistent batch-to-batch reproducibility in Mw and Purity.
- Full material traceability systems (from monomer to final polymer).
- Documented stability testing and material shelf-life validation.
- Capacity for commercial scalability and continuity of supply.
Supply Chain Risk Mitigation Strategies
Mitigating material supply risk is essential for avoiding costly line shutdowns and regulatory delays. Best practices include:
Dual Sourcing
Proactively qualifying equivalent, high-grade material from multiple vendors to eliminate single-source vulnerabilities.
Strategic Inventory
Maintaining safety stock and defined minimum/maximum buffers based on lead times and demand variability.
Comprehensive Quality Agreements
Establishing formal, detailed contracts that clearly define specifications, change management protocols, and liability transfer points between the material supplier and the manufacturer.
Conclusion
Medical-grade Polylactic Acid (PLA) is solidifying its role as the industry standard for absorbable polymers. Its core value lies in the precise tunability of its performance—from mechanical strength to degradation kinetics—achieved through controlled stereochemistry and advanced purification. This technical advantage, coupled with global regulatory imperatives for sustainable, compliant biomaterials, positions PLA as the inevitable successor to many conventional petroleum-based plastics.
Organizations aiming for market leadership in this high-growth sector must transition from general knowledge to mastery of material grades and technical sourcing requirements. Success requires aligning application-specific performance demands with the rigorous technical specifications and supply chain compliance protocols outlined in this overview. The competitive edge belongs to those who partner with suppliers guaranteeing high-purity, batch-consistent PLA raw materials.
Schedule Your Material Specification Review
Don’t risk costly delays. Book a complimentary consultation with our polymer experts to finalize your application-specific PLA grade and regulatory documentation strategy.
Frequently Asked Questions
What is PLA in medical terms?
Polylactic Acid (PLA) is a biodegradable and biocompatible thermoplastic polyester synthesized from renewable agricultural feedstocks, such as corn starch or sugarcane. In the body, it degrades via hydrolysis into lactic acid, a naturally occurring metabolite. Medical-grade PLA is strictly manufactured under cGMP conditions, ensuring superior purity, batch-to-batch consistency, and compliance with stringent ISO 10993 biocompatibility standards for use in implantable devices and advanced therapies.
What does the PLA stand for?
PLA stands for Polylactic Acid (or polylactide). The polymer structure consists of repeating lactic acid building blocks. Lactic acid monomers exist in two stereoisomeric forms: the L-isomer (Poly-L-lactic acid or PLLA) and the D-isomer (Poly-D-lactic acid or PDLA). Medical applications utilize specific ratios or blends of these isomers to precisely control desired material properties, including polymer crystallinity, mechanical strength, and the in vivo degradation rate.
Is PLA plastic food safe?
PLA holds a general recognition as food safe and possesses FDA approval for various food contact applications. Its bio-based origin and inherent biodegradability make it a preferred choice in sustainable food packaging. Nevertheless, food-grade PLA differs fundamentally from medical-grade PLA. Purity specifications, acceptable additive content, and quality control standards are significantly less stringent; medical applications strictly demand the higher purity and rigorous testing protocols specific to implantable materials.
Is PLLA better than hyaluronic acid?
PLLA (Poly-L-lactic Acid) and hyaluronic acid (HA) serve distinct, often complementary, purposes in clinical settings; direct “better or worse” comparisons are inaccurate. PLLA provides structural support and initiates collagen stimulation over a period of several months, rendering it suitable for long-term structural restoration and volume applications. Hyaluronic acid delivers immediate volume and hydration but typically exhibits faster degradation kinetics. Many facial aesthetic and regenerative strategies utilize both materials to address different aspects of tissue augmentation.
What does PLA stand for in tea bags?
PLA in tea bags indeed stands for Polylactic Acid. Many packaging manufacturers have transitioned to using PLA mesh for tea bags due to its appeal as a compostable and environmentally conscious alternative to traditional petroleum-based plastics. Importantly, this application uses commodity-grade or food-grade PLA, which possesses vastly different technical specifications and quality control requirements compared to the high-purity, batch-consistent medical-grade PLA necessary for clinical implantation.



