The clinical landscape is evolving beyond conventional petroleum-based polymers toward high-purity, bioresorbable materials that harmonize with physiological environments. Medical-grade Polyhydroxyalkanoates (PHA) represent a pivotal advancement in this transition, offering an intrinsic biocompatibility that minimizes immunological responses.
At SalesPlastics, a vertically integrated manufacturer of high-performance PHA resins with globally certified production capabilities, we specialize in tailored PHA formulations engineered to meet the stringent mechanical, thermal, and biological requirements of sophisticated biomedical engineering. By bridging the gap between ecological sustainability and clinical performance, these biopolymers enable precise advancements in long-term implantable scaffolds and targeted drug delivery systems. Our commitment goes beyond material supply—we act as a strategic co-development partner for medical device innovators, delivering consistent, high-purity custom PHA solutions that empower manufacturers to push boundaries within complex regulatory and biological frameworks.
About SalesPlastics
SalesPlastics is a premier, vertically integrated manufacturer of high-performance resins, specializing in custom medical-grade Polyhydroxyalkanoate (PHA) alongside Polylactic Acid (PLA), Polyamides (PA), and other functional polymers. With deep expertise in microbial fermentation, downstream purification, and application-specific compounding, we hold global certifications (including ISO 13485 and biocompatibility standards) and maintain rigorous supply chain consistency for demanding biomedical applications.
By integrating material science expertise with standardized quality control, we go beyond standard resins to provide fully customized PHA grades—adjusting monomer ratios, molecular weights, and additives to precisely match your device’s performance and regulatory needs. This report is designed as a technical resource for procurement partners and engineers to facilitate informed material selection and sourcing decisions.Access our complete resin product portfolio 👉
What is Medical Grade PHA?
Polyhydroxyalkanoates (PHAs) are a family of linear polyesters synthesized through microbial fermentation of sustainable feedstocks. Medical-grade PHA distinguishes itself through sophisticated downstream purification designed to eliminate residual biomass, pyrogens, and bacterial endotoxins—ensuring the material aligns with ISO 10993 safety protocols. Beyond simple biocompatibility, these biopolymers are inherently bioresorbable; their degradation byproduct, 3-hydroxybutyrate, is a natural constituent of human blood, minimizing inflammatory responses during the resorption process.
The versatility of the PHA platform allows for precise mechanical tailoring. Our specialized portfolio includes:
- PHB (Poly-3-hydroxybutyrate): Exhibits high crystallinity and tensile modulus, optimized for load-bearing orthopedic fixations.
- P34HB (Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)): A copolymer where 4HB integration disrupts polymer regularity, enhancing ductility and toughness for soft tissue scaffolds.
- P34HBHV: A ternary copolymer engineered for applications requiring enhanced optical clarity and oxidative stability, such as precision drug-eluting membranes.
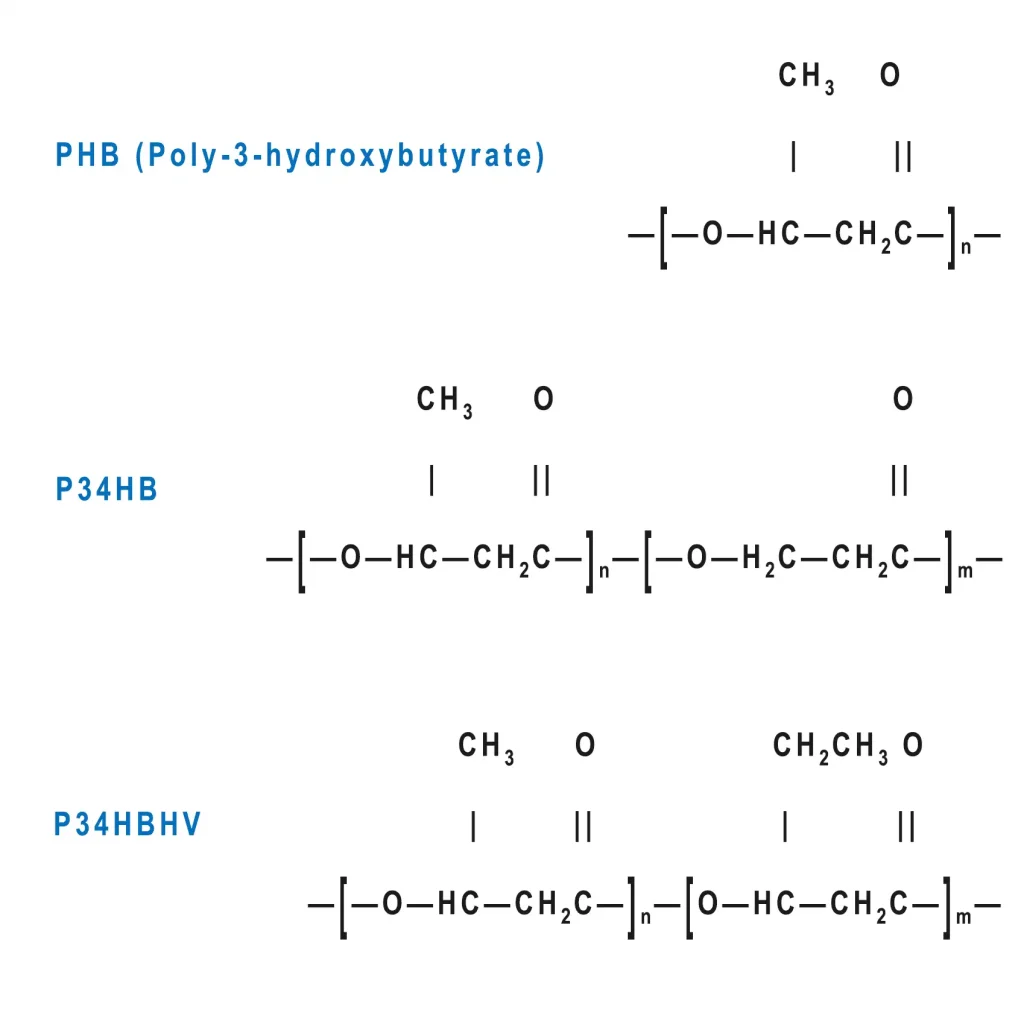
Chemical structure of PLA product portfolio
These variants support structural customization, where monomer ratios are adjusted to modulate degradation kinetics according to specific clinical healing timelines.
Medical Grade vs Pharmaceutical Grade
| Characteristic | Medical Grade | Pharmaceutical Grade |
|---|---|---|
| Primary Use | Medical devices, implants, tissue engineering | Drug formulations, excipients, delivery systems |
| Quality Standards | ISO 13485, ISO 10993 biocompatibility | USP/NF, EP, JP pharmacopeia standards |
| Testing Requirements | Biocompatibility, mechanical properties, sterilization validation | Purity, dissolution, content uniformity, stability |
| Production Environment | Clean room, controlled conditions | cGMP facilities with stringent controls |
| Regulatory Oversight | FDA medical device regulations, CE marking | FDA drug regulations, EMA requirements |
Need Technical Specifications for Medical Grade PHA?
Our team at SalesPlastics is also ready to provide detailed, application-specific information about our customized Medical Grade PHA products. Simply share your initial requirements, and we’ll respond with a personalized material recommendation within 48 hours.
Key Advantages of Medical Grade PHA
Medical-grade PHA offers a sophisticated alternative to traditional synthetic polyesters by addressing critical limitations in bio-integration and mechanical stability.
Physiological Harmony and Neutral Degradation
The primary distinction of PHA lies in its metabolic pathway. Unlike Polylactic Acid (PLA), which can induce localized metabolic acidosis due to rapid acidic byproduct release, PHA undergoes a controlled hydrolytic and enzymatic degradation. Its primary metabolite, 3-hydroxybutyric acid, is an endogenous compound within human circulation. This results in superior tissue integration and a stable peri-implant pH environment, which is vital for minimizing chronic inflammatory responses in sensitive vascular or neural applications.
Key Benefit: Medical Grade PHA degrades into non-toxic compounds that are naturally metabolized by the body, eliminating the need for removal surgeries and reducing the risk of long-term complications.
Mechanical Tunability and Processing Versatility
The structural diversity of PHA copolymers allows for the precision engineering of mechanical profiles. By modulating the monomeric ratios—such as those found in our P34HB or P34HBHV grades—the material can transition from high-modulus rigidity required for orthopedic fixation to the elastomeric ductility necessary for soft-tissue scaffolds. Furthermore, PHA maintains high thermal stability, facilitating seamless integration with existing manufacturing workflows including high-resolution 3D printing, micro-extrusion, and precision injection molding without compromising polymer integrity.
Strategic Sustainability in Healthcare
Global regulatory frameworks now place increasing emphasis on the carbon footprint of life sciences manufacturing. PHA provides a high-performance, bio-based solution derived from renewable carbon sources, effectively reducing the environmental impact of medical device production. Adopting medical-grade PHA allows manufacturers to meet emerging environmental mandates while ensuring that critical performance metrics, such as tensile strength and sterilization endurance, remain uncompromised.
Applications in the Medical Field
The structural adaptability of Medical-grade PHA facilitates its integration into diverse therapeutic modalities, ranging from transient mechanical support to biological regeneration.

Various medical applications of Medical Grade PHA
Structural Implants and Orthopedic Fixation
High-modulus PHA variants are utilized to develop bioresorbable fixation systems that synchronize with natural healing timelines.
- Orthopedic Hardware: Bone plates and screws that promote natural healing by gradually transferring mechanical loads to the bone.
- Vascular Support: Resorbable stents that provide essential support during vessel remodeling, minimizing long-term thrombosis risks.
- Wound Management: High-tensile sutures and meshes with a low inflammatory profile, ideal for complex soft-tissue closure.
Tissue Engineering & Regenerative Medicine
The surface chemistry of PHA supports cellular adhesion and proliferation, essential for tissue engineering.
- Nerve Repair: Conduct conduits that protect axonal regrowth, eliminating the need for secondary removal surgeries.
- Bio-Scaffolds: Temporary templates that facilitate the body’s transition from a synthetic matrix to natural tissue.
Cells growing on Medical Grade PHA scaffold
Advanced Drug Delivery Systems
The hydrophobic nature and crystalline structure of PHA enable precise control over the release profiles of bioactive molecules.
- Sustained Release: Microspheres that protect sensitive pharmaceuticals, ensuring a steady therapeutic dose over time.
- Smart Coatings: Device coatings customized to release anti-inflammatory agents exactly where needed.
Explore Custom Medical Grade PHA Solutions
Prefer to speak directly? Schedule a 30-minute technical consultation with our biomaterials experts—we’ll review your project and provide initial formulation suggestions at no cost.
Why Choose Customized Medical Grade PHA Supply?
Effective material integration in the medical sector relies on more than raw performance; it requires a synergy between polymer science and regulatory alignment. At SalesPlastics, our medical-grade PHA customization framework—built on vertical integration and technical co-development—focuses on three pillars of clinical success:

Tailored Material Properties
We precisely adjust molecular weight distributions, crystallinity, and copolymer ratios to achieve your target tensile strength, elongation, degradation kinetics, and biocompatibility—optimizing the material for your specific physiological environment and device requirements.

Regulatory Support
Beyond material supply, we provide essential technical dossiers, including ISO 10993 biocompatibility profiles , lot traceability, and change notification support, to facilitate streamlined submissions to notified bodies.

Supply Chain Security
For long-term medical manufacturing, consistent resin batches are vital. Our processes prioritize batch-to-batch uniformity and full-spectrum traceability to ensure patient safety and manufacturing stability.
PHA vs. PLA: Which is Better for Medical Applications?
Differentiating Polyhydroxyalkanoates (PHA) from Polylactic Acid (PLA) involves a fundamental analysis of their degradation kinetics and biological impact.
Medical Grade PHA Advantages
- Endogenous Metabolism: Degrades into 3-hydroxybutyrate, a natural human metabolite.
- pH Stability: Maintains a neutral environment, preventing localized chronic inflammation.
- Surface Erosion: Predictable, linear mass loss for reliable structural support.
- Ductile Performance: High fatigue resistance; versatile from elastomeric to rigid states.
- Thermal Stability: Robust processing across 3D printing and precision molding.
PLA Limitations
- Acidic Byproducts: Releases lactic acid, often inducing localized metabolic acidosis.
- Bulk Erosion: Prone to sudden, non-linear loss of mechanical integrity.
- Autocatalytic Effect: Accelerated degradation that complicates healing timelines.
- Inherent Brittleness: High risk of premature structural failure under physiological stress.
- Process Sensitivity: Narrow thermal window and high susceptibility to moisture.
While PLA is cost-effective for certain uses, PHA excels in regenerative applications where biocompatibility is paramount.
Challenges and Future Prospects
The transition of Medical-grade PHA from specialized research to mass-market clinical adoption involves several critical technical hurdles that define current innovation priorities.
Current Challenges
Scalability and Economic Feasibility
The high purity requirements of medical-grade fermentation drive costs above those of bulk polymers. Research focuses on strain engineering and advanced solvent-free extraction to optimize yields.
Sterilization Validation
Establishing standardized protocols for Gamma, EtO, or E-beam sterilization that maintain polymer chain integrity without compromising mechanical performance.
Processing Constraints
The narrow thermal processing window of certain PHA variants necessitates specialized additive manufacturing or precise compounding to prevent thermal degradation during production.
Long-term Clinical Evidence
While acute biocompatibility is verified, longitudinal data regarding the late-stage remodeling of PHA-based implants remains a primary focus of ongoing clinical trials.
Future Prospects

Next-generation applications of Medical Grade PHA in development
The future of Medical Grade PHA in healthcare looks promising, with several exciting developments on the horizon:
- 3D Bioprinting: Formulation of PHA-based bio-inks to facilitate the extrusion of complex, cell-laden architectures for vascular and organ tissue engineering.
- Smart Materials: Development of stimuli-responsive PHA matrices capable of localized property shifts in response to physiological pH or temperature triggers.
- Pharmacological Integration: Engineering of multifunctional devices that combine structural support with the controlled release of growth factors or anti-microbial agents.
- Patient-Specific Implants: Leveraging PHA’s customizable degradation and 3D printability to create patient-matched scaffolds for complex reconstructive surgery.
Conclusion
Medical-grade PHA signifies a pivotal shift toward bioresorbable therapeutic architectures that harmonize physiological safety with structural precision. By integrating intrinsic biocompatibility with customizable degradation kinetics, these biopolymers provide a robust framework for advanced tissue engineering and targeted pharmacological delivery.
While the path toward large-scale standardization involves ongoing technical refinement, the empirical evidence supporting PHA’s clinical efficacy continues to expand. For manufacturers and researchers seeking a reliable partner in medical-grade biomaterials, SalesPlastics offers high-purity, custom PHA variants—including PHB, P34HB, and P34HBHV—developed in close collaboration to meet your exact mechanical, biological, and regulatory specifications.
We invite you to engage with our technical team for a complimentary initial consultation, material recommendation, or sample evaluation—ensuring your next-generation medical device leverages the full potential of tailored PHA technology. Contact us today to start the co-development process.

Advancing healthcare through Medical Grade PHA research and development
Ready to Integrate Medical Grade PHA Into Your Products?
Contact our team of specialists to discuss your specific requirements and discover how Medical Grade PHA can enhance your medical device performance.
Frequently Asked Questions
What are medical grade products?
Medical-grade products are materials and components engineered to meet rigorous safety benchmarks, such as ISO 13485 for quality systems and ISO 10993 for biological evaluation. These products are characterized by high purity, documented batch traceability, and comprehensive biocompatibility testing. This ensures their safety within specific clinical risk profiles, ranging from transient external contact to permanent physiological implantation.
What is a medical grade polymer?
A medical-grade polymer is a high-purity resin specifically formulated and certified for clinical safety. Unlike industrial polymers, these materials are produced in controlled environments to eliminate chemical or particulate contamination. They must demonstrate consistent physical properties after sterilization and comply with regulatory standards like USP Class VI or ISO 10993 to ensure no adverse systemic or localized reactions occur during use.
What is a PHA in medical terms?
In medical terms, PHA (Polyhydroxyalkanoate) refers to a family of microbial-derived polyesters synthesized through bacterial fermentation. They are valued for their bioresorbability and mechanical tunability. Medical-grade PHA undergoes advanced purification to remove residual proteins and bacterial endotoxins, ensuring its degradation byproduct—3-hydroxybutyric acid—is safely metabolized as a natural constituent of human blood.
Which is better, PLA or PHA?
The selection between PLA and PHA depends on the functional requirements of the specific application:
- PHA is preferred for regenerative medicine where pH-neutral degradation and superior tissue integration are critical to prevent localized inflammation.
- PLA remains an industry standard for applications requiring high stiffness and established high-volume manufacturing protocols. While PLA is cost-effective, PHA offers a more versatile range of ductility and a degradation profile that aligns more closely with natural physiological processes.
How is Medical Grade PHA sterilized?
Sterilization protocols must be validated to ensure the maintenance of polymer chain integrity:
- Ethylene Oxide (EtO): The primary method for PHA, as it utilizes lower temperatures that preserve the material’s molecular weight and mechanical properties.
- Radiation (Gamma/E-beam): Applicable but requires precise dose control to mitigate potential chain scission, which may inadvertently accelerate degradation rates.
- Steam (Autoclaving): Generally avoided for most PHA formulations due to the risk of thermal deformation and uncontrolled hydrolytic degradation.





