The healthcare industry faces an escalating dilemma: balancing the non-negotiable requirement for sterile, high-barrier medical packaging with intensifying environmental mandates. Traditional petroleum-based polymers contribute significantly to the sector’s carbon footprint; Current industry estimates suggest that a significant portion — often cited between 20% and 30% — of hospital waste consists of plastic packaging and products.
As global healthcare systems align with ESG goals, Medical Grade Bioplastic Resins have emerged as a disruptive solution . These Sustainable Medical Packaging Materials offer an alternative that upholds rigorous mechanical and microbial barrier standards for Class I, II, and III medical devices, while enabling a healthcare circular economy.
For readers seeking a broader understanding of starch-based biodegradable materials — including their composition, manufacturing processes, and real-world commercial applications — you may refer to our detailed guide, “Starch-Based Biodegradable Plastics: Materials, Applications, and Commercial Reality.”
Pain Points of Traditional Sterile Medical Packaging

Traditional plastic medical packaging contributes significantly to healthcare waste streams
The medical packaging industry has long relied on petroleum-based polymers such as Polyethylene Terephthalate (PET), Polyvinyl Chloride (PVC), and Polypropylene (PP). While these materials offer high barrier properties, their lifecycle limitations are creating a strategic bottleneck for modern healthcare.
Environmental Liability
Traditional packaging takes centuries to decompose. With healthcare generating nearly 6 million tons of waste annually in the U.S. alone, the sector is under fire for contributing to the global microplastic crisis.
Carbon & Scope 3 Risks
From fossil fuel extraction to incineration, traditional plastics are carbon-intensive. For organizations aiming for Net Zero, these materials are the primary obstacle to reducing Scope 3 emissions.
Regulatory "Tsunami"
New mandates like the EU’s PPWR and California’s SB 54 are moving beyond suggestions to strict requirements. Non-compliance now means market access risks and heavy EPR (Extended Producer Responsibility) fees.
Operational Hidden Costs
Managing high-volume plastic waste isn’t just an environmental issue—it’s an expensive logistical burden involving complex segregation and rising disposal fees.
Understand Your Medical Packaging Environmental Impact
Use our Medical Packaging Sustainability Audit to evaluate your environmental footprint and discover how switching to Starch-Based Resins can mitigate regulatory fees.
Technical Evolution of Starch-Based Degradable Plastics

Research and development of advanced starch-based polymers for medical applications
The transformation of starch into a robust medical-grade resin is the result of sophisticated macromolecular engineering. The focus is on providing “Drop-in” solutions that require minimal adjustment to existing polymer compounding and extrusion lines.
High-Purity Extraction for Biocompatibility
Unlike industrial-grade starch, our medical-grade feedstocks undergo multi-stage wet milling and purification. This ensures the removal of proteins and endotoxins, meeting the stringent biocompatibility requirements for sterile barrier systems.
Molecular Engineering (Esterification & Grafting)
Native starch is hydrophilic; however, through Acetylation and Grafting (with PLA/PCL), we create a thermoplastic matrix with superior moisture resistance. This ensures that the packaging maintains its tensile strength and barrier integrity even under the high-humidity conditions often encountered during EO (Ethylene Oxide) sterilization.
Advanced Compounding & Blending
Our resins are not simple starches but advanced alloys. By blending with PBAT for flexibility or PHAs for water resistance, we achieve a “balance of properties” that allows for:
- High Puncture Resistance: Essential for sharp medical instruments.
- Optical Clarity: Allowing for visual verification of device integrity.
- Thermal Stability: Ensuring seal strength remains consistent across global supply chains.
Nanotechnology
The integration of Montmorillonite nanoclays creates a “tortuous path” for gas molecules.This provides an Oxygen Transmission Rate (OTR) that rivals traditional PET/PE laminates, essential for protecting oxidation-sensitive medical components.
Performance Comparison: Starch-Based Plastics vs. Traditional Plastics

Laboratory performance testing comparing traditional and starch-based packaging materials
To serve as a viable alternative, starch-based resins must satisfy the rigorous safety margins of sterile barrier systems. Below is a head-to-head comparison of our high-performance formulations against traditional medical polymers.
Mechanical Properties & Durability
Our modified starch alloys minimize the performance gap, ensuring structural integrity from the production line to the clinical environment.
| Property | Traditional Plastics (PET/PP) | Starch-Based Resins (Medical Grade) | Application Impact |
| Tensile Strength (MPa) | 40 – 60 | 38 – 52 | Structural support for kits |
| WVTR (g/m²/day) | 0.5 – 5.0 | 2.5 – 8.0 | Moisture barrier integrity |
| OTR (cc/m²/day) | 30 – 100 | 15 – 50 | Superior gas barrier |
| Puncture Resistance (J/m) | 10 – 15 | 11 – 14 | Protection against sharps |
Sterilization Compatibility Matrix
Sterilization is the “litmus test” for medical packaging. Our starch-based grades are engineered to maintain macromolecular stability across the most common industry methods.
Sterilization Compatibility Strengths
- Ethylene Oxide (EtO): Excellent stability; the preferred method for starch-based barrier systems.
- Gamma Irradiation: Compatible at standard doses (25 kGy). For higher doses, we provide specialized radiation-stabilized formulations to prevent yellowing or embrittlement.
- E-Beam: Suitable with minimal property changes.
Sterilization Compatibility Limitations
- High-Temperature Steam (>121°C): Traditionally limited; however, our “High-Heat” series is designed for short-cycle autoclaving.
- Dimensional Stability: [ADDED] We provide pre-validated shrinkage data to assist in mold design and ensure seal integrity post-sterilization.
The compatibility of starch-based plastics with ethylene oxide sterilization—the most widely used method for temperature-sensitive medical devices—represents a significant advantage for their adoption in the medical packaging sector. Specialized formulations have also been developed for applications requiring steam sterilization, though these typically incorporate higher proportions of heat-resistant copolymers.
Shelf Life and Stability
Long-term stability is essential for medical packaging, which must maintain integrity throughout the product’s shelf life. Modern starch-based plastics have overcome many of the early stability challenges:
- Ambient Storage Stability: Advanced formulations maintain mechanical and barrier properties for 2-3 years under controlled ambient conditions (20-25°C, 40-60% RH)
- Accelerated Aging Performance: Testing at elevated temperatures (40°C, 75% RH) demonstrates stability comparable to conventional plastics for periods equivalent to 18-24 months of real-time aging
- Microbial Resistance: Incorporation of natural antimicrobial compounds provides inherent protection against biofilm formation and microbial growth
- UV Stability: Addition of natural UV stabilizers derived from plant extracts provides protection against photodegradation during storage
While the shelf-life capabilities of starch-based plastics have improved dramatically, they typically require more controlled storage conditions than their petroleum-based counterparts, particularly with respect to humidity control. This represents an area of ongoing research and development.
Compare Material Performance for Your Application
Access our detailed comparison matrix of starch-based vs. traditional plastics across all critical performance parameters for medical packaging.
Applications of Starch-Based Degradable Plastics in Medical Packaging
Medical devices packaged in various starch-based degradable plastic formats
Our starch-based resins are not just “alternatives”—they are engineered alloys designed for high-performance medical conversion.
Sterile Barrier Pouches & Flexible Packaging
These grades are optimized for multilayer lamination, typically serving as the structural or sealant layer.

- Sealing Performance: Provides a wide heat-sealing window and consistent seal strength, ensuring integrity during pressure changes in air-freight.
- Aseptic Opening: Designed for a clean, fiber-free peel, preventing particulate contamination during device extraction in the OR.
Rigid Trays & Blister Packaging
We have addressed the “snap-back” and brittleness issues often associated with earlier biopolymers.

- Deep-Draw Capabilities: Excellent melt strength allows for complex tray geometries without thinning or “webbing.”
- Impact Resistance: Specifically formulated to protect heavy orthopedic implants or delicate catheter systems during 1-meter drop tests.
Functional & Smart Packaging
Antimicrobial Integration
The porous nature of the starch matrix can be leveraged to carry natural antimicrobial agents, creating an active sterile barrier.
Tamper Evidence
High-clarity films that exhibit distinct stress-whitening upon opening, providing a built-in safety feature for patients.
Begin Your Sustainable Packaging Journey
Take the first step toward implementing Medical Grade Bioplastic Resins in your medical packaging applications.
Advantages of Starch-Based Degradable Plastics
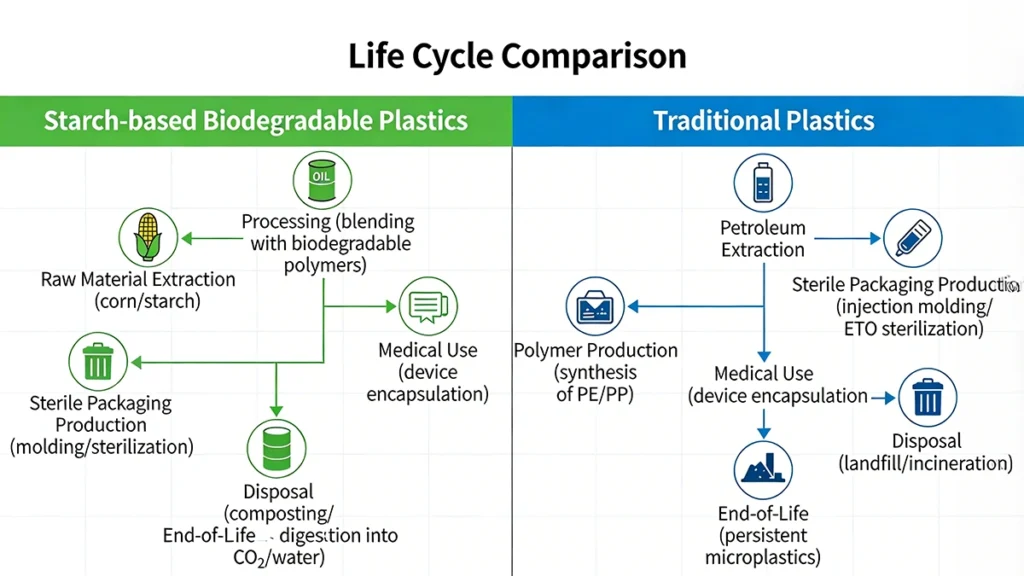
Life Cycle Comparison: Starch-Based Biodegradable Plastics vs. Traditional Plastics in Medical Sterile Packaging
The transition to starch-based degradable plastics for sterile medical packaging offers a multi-dimensional value proposition. Beyond simple material substitution, these innovative resins provide a strategic pathway to meet environmental mandates while enhancing operational resilience and patient safety.
Environmental Stewardship & Carbon ROI
Starch-based plastics offer a significantly lower environmental impact compared to conventional petroleum-based polymers.
- Decarbonization: Life Cycle Assessment (LCA) data indicates that starch-based resins typically generate 50-70% less greenhouse gas emissions. By utilizing carbon-sequestering plant feedstocks, manufacturers can drastically reduce their Scope 3 emissions.
- Renewable Sourcing: Moving away from finite fossil fuels, our resins utilize annually renewable agricultural resources, supporting a regenerative “cradle-to-gate” manufacturing model.
Certified Biodegradability and Waste Management
One of the most critical advantages for the healthcare sector is the elimination of long-term plastic persistence and microplastic pollution.
- True Microbial Assimilation: Unlike oxo-degradable plastics that merely fragment, our starch-based materials are fully consumed by microorganisms, leaving zero microplastics or toxic residues.
- Standard Compliance: Our medical-grade formulations are engineered to meet the most stringent international standards for compostability and biodegradability:
| Environment | Degradation Timeline | End Products | Regulatory Standard |
| Industrial Compost | 90 – 180 Days | CO2, H2O, Biomass | EN 13432 / ASTM D6400 |
| Home Compost | 180 – 365 Days | CO2, H2O, Biomass | TÜV OK Compost Home |
| Soil Burial | 1 – 2 Years | Nutrients & Humus | ISO 17556 |
Regulatory Compliance and Future-Proofing
As global packaging regulations tighten, starch-based materials provide a “compliance shield” for medical device manufacturers (MDMs).
Mitigating Plastic Taxes
Use of certified compostable and bio-based content helps organizations avoid or reduce escalating Plastic Packaging Taxes in the EU, UK, and various US states (e.g., California’s SB 54).
EPR Incentives
Many jurisdictions offer lower Extended Producer Responsibility (EPR) fees for packaging that integrates into circular waste streams, directly impacting the bottom line.
Superior Biocompatibility and Patient Safety
In the medical field, material purity is as vital as environmental performance.
- Chemical Purity: Our starch resins are inherently BPA-free, Phthalate-free, and PFAS-free. This eliminates the risk of endocrine-disrupting chemicals leaching into sensitive devices or pharmaceuticals.
- Ultra-Low Extractables: The natural origin of the feedstock ensures a minimal chemical footprint, ensuring compliance with USP Class VI and ISO 10993 biocompatibility standards.
Economic Logic and Brand Differentiation
While resin costs may be higher, the Total Cost of Ownership (TCO) is often optimized through:
- GPO & Tender Advantages: Sustainability is now a weighted criterion in hospital Group Purchasing Organization (GPO) contracts. Eco-friendly packaging acts as a powerful brand differentiator.
- Waste Operational Savings: By diverting non-contaminated packaging from high-cost “Red Bag” incineration to composting streams, healthcare facilities can realize significant disposal savings.
Challenges in the Adoption of Starch-Based Plastics

Research and development efforts focused on addressing technical limitations of starch-based materials
While the shift to starch-based materials involves certain complexities, these are manageable hurdles with the right technical partnership.
- Technical Resilience: To counter starch’s natural hydrophilicity, our latest grades utilize Hydrophobic Grafting and multilayer co-extrusion. This ensures stable performance and ISO 11607 compliance across variable climates.
- Processing Ease: We offer formulations with a stabilized thermal profile and a wider processing window. Our team provides on-site processing optimization to ensure your existing thermoforming lines run smoothly with minimal recalibration.
- Sterilization & Validation: Our materials excel in EtO and Gamma (up to 25kGy) environments. We simplify the daunting ISO 10993 and USP Class VI navigation by providing a comprehensive Technical Dossier for every grade, drastically reducing your time-to-market.
- The Economic Lens: The “cost premium” is rapidly diminishing. When factoring in Plastic Tax offsets, lower EPR fees, and the ability to down-gauge due to high material stiffness, the total cost of ownership becomes highly competitive.
Future Trends in Starch-Based Degradable Plastics
The field of starch-based plastics is evolving from “simple biodegradable bags” to “high-performance medical systems.” As a distributor, we see three critical trends shaping the next generation of starch-based raw materials:
- Nanotechnology Integration: Next-generation resins reinforced with Cellulose Nanocrystals (CNCs) now create a “tortuous path” for gas molecules, enhancing moisture barriers by up to 80% without sacrificing compostability.
- Active & Intelligent Packaging: Starch-based matrices are becoming “active” carriers for natural antimicrobials and oxygen scavengers, while integrated Time-Temperature Indicators (TTI) provide visual alerts if the sterile chain is compromised.
- Regulatory-Driven Circularity: Future formulations focus on agricultural waste-feedstock, ensuring medical packaging aligns with the strict ESG goals of modern healthcare systems while maintaining full biocompatibility.
Conclusion

Healthcare facilities increasingly adopting starch-based degradable plastics as part of comprehensive sustainability initiatives
Starch-based degradable plastics have transitioned from a promising sustainable concept into a high-performance benchmark for sterile medical packaging. As the healthcare industry accelerates its transition toward carbon neutrality, these materials offer a critical synergy between uncompromising patient safety and environmental stewardship.
Our technical evolution has proven that starch-based resins are no longer “compromise materials.” Through advanced chemical modification and nanocomposite integration, our raw materials now deliver the mechanical strength and barrier properties—specifically oxygen and moisture resistance—required for the most demanding clinical environments. These bio-based solutions allow healthcare organizations to meet their ESG (Environmental, Social, and Governance) targets while ensuring full alignment with ISO 10993 biocompatibility and EN 13432 compostability standards.
While traditional starch materials faced hurdles like moisture sensitivity, our current portfolio of engineered biohybrid resins addresses these challenges head-on. These materials are optimized for high-speed production on existing conversion lines, ensuring cost-effective scalability and minimal operational disruption for medical device manufacturers.
Looking ahead, we are not just providing a substrate, but a platform for innovation. From active packaging that extends shelf-life to integrated device-packaging systems, our starch-based resins are designed to power the next generation of medical technology.
Starch-based resins are no longer “experimental materials”—they are proven, high-performance solutions for the modern medical landscape. By choosing our engineered resins, you gain a strategic partner dedicated to navigating the complex intersection of material science, global regulation, and environmental responsibility.




